Functional Groups in Organic Chemistry
What are Functional Groups?
-
In terms of organic chemistry, functional groups are atoms and groups of atoms present in molecules that give the molecule a specific reactivity.
-
Specific functional groups tend to undergo the same or very similar reactions regardless what molecule they are found on.
Below is a diagram showing the most common functional groups presented in university courses. It is not a complete list of every functional group that exists but rather just the ones found most commonly in second-year organic chemistry courses. "R" is a placeholder, which essentially denotes the rest of the molecule.

When learning functional groups, we can make our lives easier by identifying patterns, just like with chemical reactions. Here are some trends we can identify to lessen the amount of information we need to memorize:
-
An alcohol differs from a thiol group only in that the O is replaced with an S. The same applies to an ether and thioether.
-
Aldehydes and ketones are almost the same except that the H in the aldehyde is replaced by another R group.
-
Imines are similar to aldehydes and ketones, except the carbonyl oxygen in the aldehydes and ketones is replaced with a nitrogen.
-
Oximes and hydrazones are similar to imines, except the H or R groups on imines are replaced by an OH or an NH2.
-
Ester, acid anhydride, acid halide, and amide functional groups are all carboxylic acid derivatives. The R-(CO) is common to all of them, with only the second substituent on the carbonyl carbon being different.
Knowing the relative electronegativities of various atoms in each functional group can make it easier to predict reactivity. Patterns based on electronegativities may be useful in determining which functional groups may be acidic or basic, nucleophilic or electrophilic.
Functional Group: Alkanes

-
Alkanes are the simplest of the hydrocarbon functional groups.
-
Alkanes contain only single bonds and are said to be fully-saturated.
-
All carbon atoms in alkanes are sp3-hybridized.
-
The geometry around each carbon is tetrahedral.
-
The bond angles are 109.5º.
-
-
They are non-polar.
-
They are essentially non-acidic and non-basic.
-
The boiling points of alkanes depend on London Dispersion Forces and how well the molecules can 'pack', which also depends on whether they are linear, branched, the degree of branching, etc.
Alkanes are fairly inert and unreactive overall. Their main reactions are combustion (burning) and radical halogenations, usually involving radical initiators or UV light.
Functional Group: Alkenes

-
Alkenes are the first hydrocarbon functional group which exhibits a much more comprehensive range of reactivity.
-
Alkenes contain double bonds between two carbon atoms.
-
Carbon atoms in alkenes are sp2-hybridized.
-
Each double bond is comprised of a sigma bond and a pi bond.
-
The geometry around each carbon atom is trigonal planar.
-
An outline of the most common alkene reactions and mechanisms can be found here.
Functional Group: Alkynes

-
Alkynes are the most unsaturated hydrocarbon functional group.
-
Alkyne carbon atoms are sp-hybridized.
-
Alkynes are linear.
-
-
Terminal alkynes contain a hydrogen atom at one end of the alkyne.
-
The hydrogen atom is weakly-acidic, with a pKa of ~25.
-
The hydrogen atom can be removed with a strong base, such as sodium amide or alkyllithium.
-
An outline of the most common alkyne reactions, syntheses, and mechanisms can be found here.
Functional Group: Arenes

-
Arenes are comprised of cylic, conjugated hydrocarbons.
-
Double bond electrons are delocalized in a pi cloud that's parallel to the plane of the ring.
-
-
Arenes are aromatic.
-
Carbon atoms in arenes are sp2-hybridized and their geometry is trigonal planar, similar to alkenes.
-
The simplest arene is benzene.
-
Methylbenzene is commonly known as toluene.
-
Disubstituted arenes are designated as ortho when they are 1,2-disubstituted, meta when 1,3- and para when they are 1,4-disubstituted.
-
An outline of the most common arene reactions, syntheses, and mechanisms can be found here.
Functional Group: Alkyl Halides
-
Alkyl halides are composed of:
-
Alkyl fluorides (R-F)
-
Alkyl chlorides (R-Cl)
-
Alkyl bromides (R-Br)
-
Alkyl iodides (R-I)
-
-
Alkyl halides are
-
electrophilic at the carbon bonded to the halogen atom.
-
important substrates in substitution and elimination reactions.
-
often be synthesized directly from alkenes and alcohols.
-
Functional Group: Alcohols and Thiols
-
The C-O bond in alcohols is very polar since oxygen is more electronegative than carbon.
-
Alcohols can be deprotonated using bases to form alkoxides, which can be basic, nucleophilic, or both.
-
Alcohols participate in hydrogen bonding and therefore have higher boiling points than non-polar hydrocarbons.
-
Alcohols may be directly synthesized from alkenes, alkyl halides, among other functional groups.
-
Alcohols may be reacted with alkyl halides, in the presence of bases, in the Williamson ether synthesis in order to form ethers.
An outline of the most common alcohol reactions, syntheses, and mechanisms can be found here.
-
Thiols are analogous to alcohols except they have sulfur instead of oxygen.
-
Thiols behave similarly to alcohols except sulfur is a larger atom than oxygen, in the same group (column) of the periodic table.
-
Thiolates are less basic but more nucleophilic than alkoxides due to the electronegativity differences between oxygen and sulfur.
-
Similar to alcohols, thiols may be deprotonated and reacted with alkyl halides in order to form thioethers (sulfides).
Functional Group: Amines

-
Amines consist of nitrogen bonded to either hydrogen or carbon atoms.
-
The nitrogen atom may be either sp2 or sp3-hybridized.
-
If the carbon atoms bonded to nitrogen contain only single bonds, the nitrogen is sp3-hybridized.
-
If any of the carbon atoms bonded to the amine nitrogen have double bonds, the nitrogen will be sp2-hybridized.
-
-
The N-H bond is very polar and amines are capable of hydrogen bonding, which increases their water solubilities and boiling points.
An outline of the most common amine reactions, syntheses, and mechanisms can be found here.
Functional Group: Ketones, Aldehydes, Imines, Oximes, and Hydrazones

-
The C=O group is called a carbonyl.
-
Ketones, aldehydes, imines, oximes, and hydrazones fall under the category of carbonyl-containing functional groups.
-
Aldehydes have one alkyl group bonded to the carbonyl carbon whereas ketones have two alkyl groups
-
The C-H bond in aldehydes is polar-covalent but aldehydes are not hydrogen bond donors.
-
-
Imines, oximes, and hydrazones are formed by reacting amines, hydroxylamine, or hydrazines with ketones or aldehydes in the presence of acid.
-
All of the above functional groups have in common that they are electrophilic at the carbonyl carbon, to varying degrees.
An outline of the most common ketone and aldehyde reactions, syntheses, and mechanisms can be found here.
Functional Group: Acids, Esters, Amides, Anhydrides, and Acid Halides
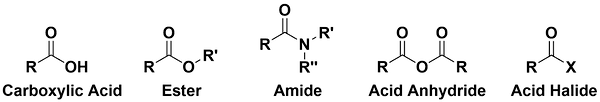
-
Carboxylic acids and their derivatives also contain the C=O carbonyl functional group but they behave differently than aldehydes, ketones, and their derivatives.
-
The oxygen or nitrogen bonded to the C=O is able to donate its electrons into the carbonyl through resonance, thereby decreasing the electrophilicity of the carbonyl carbon.
-
-
Unlike ketones and aldehydes, carboxylic acids and their derivates are able to undergo addition-elimination reactions in the presence of strong nucleophiles, such as organolithium reagents (R-Li).
-
As the name suggests, carboxylic acid derivatives may be synthesized from carboxylic acids through various reactions.
-
Esters contain an alkoxy group bonded to the carbonyl carbon.
-
Amides contained an amino group bonded to the carbonyl carbon.
-
Acid anhydrides contain a carboxylate bonded to a carbonyl carbon.
-
Acid halides contain a halogen atom bonded to a carbonyl carbon.
-
-
Order of reactivity:
-
Acid halide > Acid Anhydride > Ester > Carboxylic Acid > Amide > Carboxylate (COO-)
-
Functional Group: Nitriles, Azides, and Nitros
-
In terms of Organic I and Organic II, nitro functional groups are seen most often on aromatic rings, although they also exist on sp3-hybridized carbons as well, as seen in nitromethane.
-
Nitriles are composed of an sp-hybridized nitrogen triple-bonded to an sp-hybridized carbon
-
The nitrile functional group is also often referred to as a "cyanide"
-
-
Azides contain three nitrogen atoms bonded to each other and the terminal nitrogen bonded to a carbon atom.
-
Molecules containing azides are often unstable and explosive.
-
You've made it to the end! These are only the most common functional groups covered in organic I and II university-level courses. Many more exist, although they are similar in nature to the ones shown and their reactivities bearing resemblance as well.
Want to see other functional groups not mentioned here? Let me know!